Last modified: 28 February, 2022
Introduction
Send this intervention to your inbox
This is a guide to dried blood spot (DBS) testing for hepatitis C (HCV). This guide provides an overview of how DBS testing works and how you can implement it in your service setting. We’ll give you some practical tools, tips, and resources and provide insights into the barriers you may face and how you might be able to overcome these barriers.
The implementation or scaling up of DBS testing is country and/or region-specific and the information we have provided is of a general nature.
The authors and reviewers of this guide include healthcare practitioners, researchers, laboratory scientists, policy makers and other service providers globally. This guide is part of our Intervention Toolkit which profiles innovative models of HCV care and offers guidance on how to set up different interventions for HCV in your service.
Definition
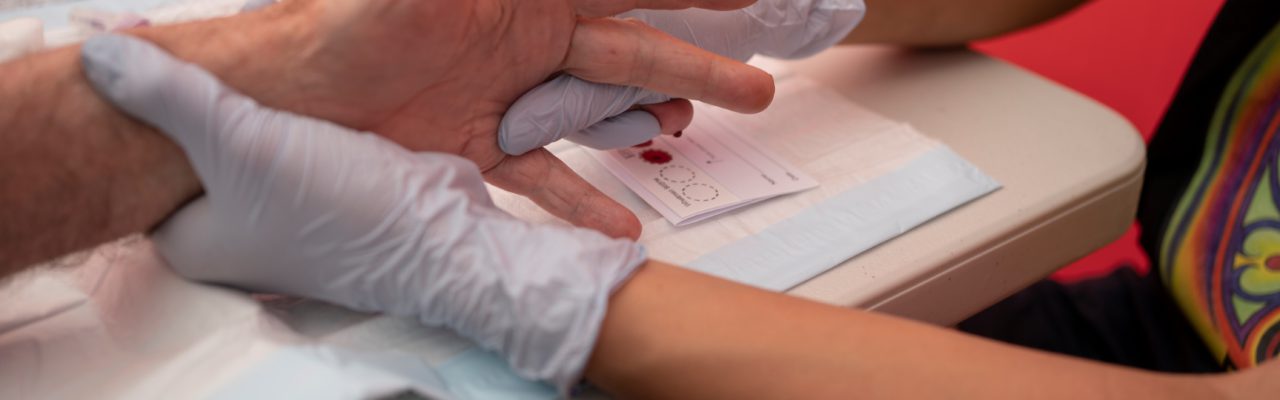
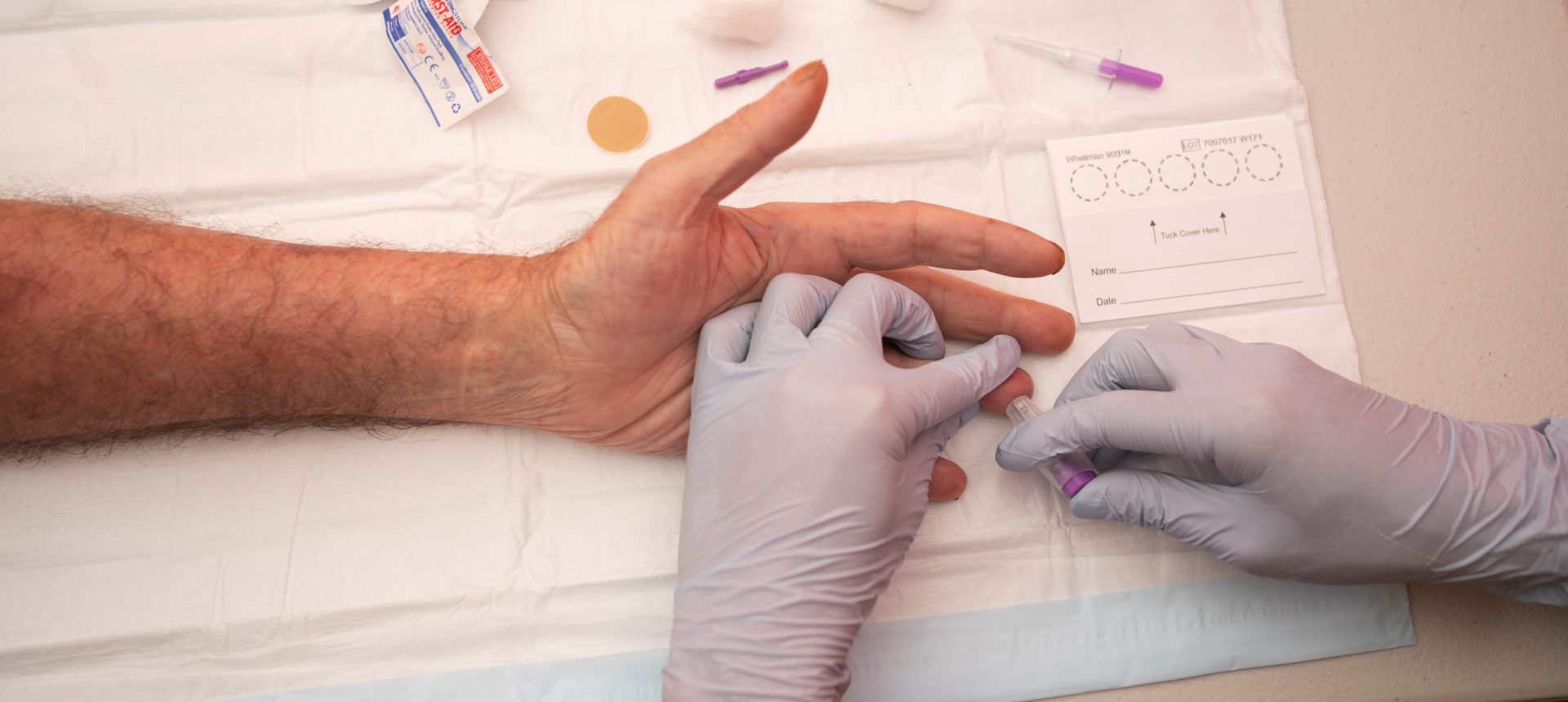
Dried Blood Spot (DBS) testing is a blood collection method that simplifies the HCV testing process.
DBS testing involves collecting capillary blood samples from a finger prick on filter paper which is dried before it’s sent to a lab for testing.
It can enhance your model of care by reducing the barriers to collecting blood for testing, including:
- difficult venous access
- the need to centrifuge blood samples following collection
- the need to ship blood using cold-chain transportation
DBS can also be used to test for other infectious diseases, including (but not limited to) human immunodeficiency virus (HIV) and hepatitis B virus (HBV).
How does DBS testing work?
To perform the test, we prick the finger with a lancet and then spot the blood on to a card made of filter paper, making sure the finger doesn’t touch the card (see INHSU – How to collect a DBS sample). Once dry, the card is sent to a lab for testing.
Some labs will test for HCV antibodies and, if positive, they will they test for HCV ribonucleic acid (RNA). Other labs will go straight to HCV RNA testing – this often differs by region and target population according to the observed HCV prevalence.
Anyone can collect a dried blood spot sample if properly trained. This means clients and people who are not health practitioners (such as peer support workers, community service workers) can support HCV testing.
- It can be done wherever the person is – either at home, or in another setting, such as a drug rehabilitation centre, NSP, pharmacy, or prison.
- Samples can be self-collected or collected by peers or other people who are not healthcare providers, with specific training.
- It reduces the need for venepuncture, which is helpful for people with poor venous access.
- It facilitates access to testing for people in regional and remote areas who may not have easy access to healthcare facilities.
- It’s easy to store and transport collected samples, as they are stable at room temperature once dry for approximately two weeks when stored with desiccant. This allows for ambient storage and postage.
- Both HCV antibodies (to check for previous exposure to the virus) and HCV RNA (to confirm viremic infection) can be detected from DBS.
- HIV and other viruses can be tested on a DBS card. Availability of testing for other diseases will depend on your region.
-
It works to reduce stigma and discrimination by offering a low threshold testing option either at home or in places and services where clients already feel comfortable or already attend.
- As only a few drops of blood are collected and dried, a reduced sensitivity of the laboratory assays may be expected when using DBS instead of venous blood. This means that if very low levels of virus (RNA) are present, they might not be detected from DBS. However, a lower sensitivity may be accepted if an assay is able to improve access (such as those based on DBS specimens) and/or affordability; according to the EASL testing guidelines, for widespread population testing, a qualitative HCV RNA assay needs only to have a lower limit of detection of ≤1000 IU/mL1.In such settings, the low incidence of a false-negative test for viraemia is outweighed by the benefit of scaling up access to diagnosis and care.
- Very humid environments can sometimes affect the DBS sample, though this is rare. DBS cards are usually shipped in a gas impermeable bag with desiccant packs to reduce any potential moisture damage to the sample, and a humidity card to indicate any moisture damage.
- The supplies for DBS testing may not be publicly funded in some regions.
- Collection experience makes a difference to sample quality. When the lancet is used properly, it’s possible to collect an adequate sample without multiple punctures. Obtaining the sufficient amount of blood is especially important when testing for the presence of the virus (RNA), rather than just antibodies.
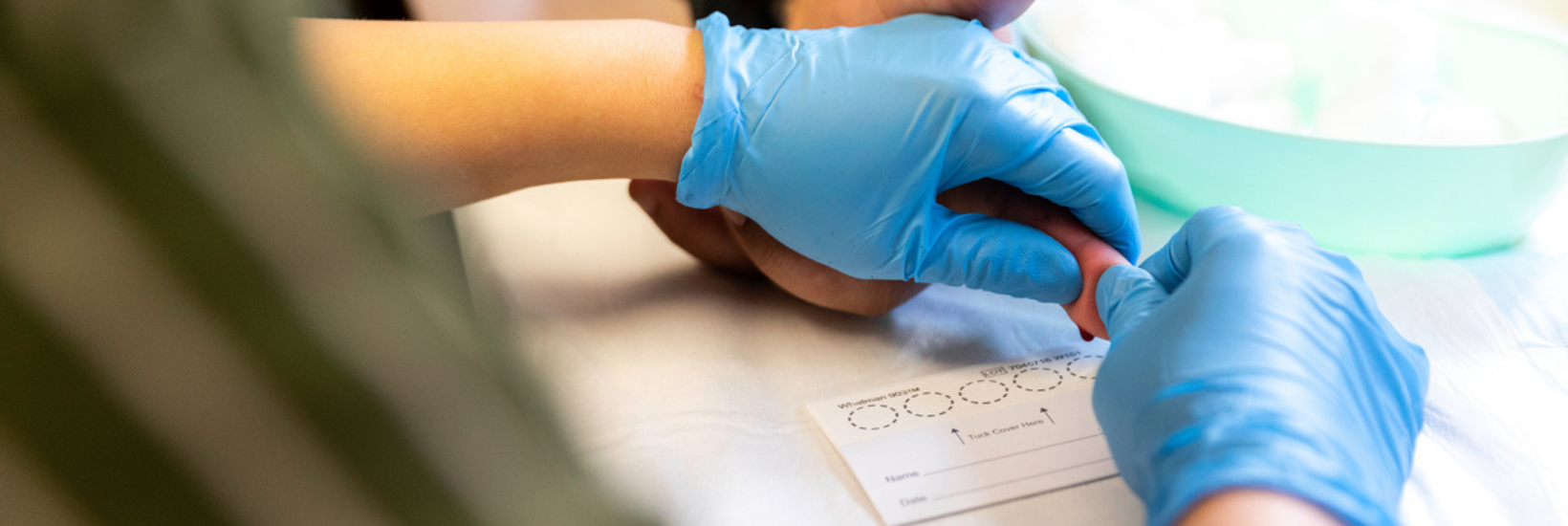
How to set up DBS testing in your service
Availability: Check whether DBS testing is available in your region and whether it is approved for use for HCV. Contact your local health department or local microbiology/pathology provider to find out more.
Postage: Ask your postal service whether they permit postage of the DBS collected samples. If not, seek their permission to do so. DBS samples are usually exempt from infectious substances classifications, so can be posted without issues. Check with your local microbiology/pathology provider if you are unsure.
Helpful resources:
DBS kit supplier: Identify a test kit supplier – ask whether they allow for client self-collection, or if there is a requirement for medically trained staff to perform the collection. DBS sample collection kits sometimes come with all the items needed for the test, including instructions. If not, you can order the separate components of the test kit and make your own.
Laboratory to process your samples: Ask your microbiology/pathology provider, or ask your local health department for guidance. Once identified, talk to a lab to arrange for the completed sample to be posted or delivered to the lab for processing.
Other suppliers: Identify the suppliers of items you might need if they do not come in the kit, such as lancets, swabs, adhesive bandages, disinfectant, and biohazard/sharp containers if capillaries or minivettes are used.
Helpful resources:
Key stakeholders: Identify people in your organisation who need to agree to adopt DBS testing.
Evidence: Write a list of reasons why they should support this initiative – identify the benefits.
Partnerships: Consider partnerships with a range of stakeholders, would any of these stakeholders support your plan? In what way?
Elimination goals: Consider linking DBS testing for HCV to any state, national or international strategic hepatitis elimination plans or goals
Risk management: Show your key stakeholders that you have thought about the risks. Identify any possible risks and demonstrate how you will manage them.
Budget: Put together a budget to show how much it will cost. Consider comparing the cost per DBS test to traditional testing methods.
Infrastructure: Identify what additional equipment and space you will need. Is there existing infrastructure you could use?
Staff: Consider how you will staff the addition of DBS testing to your model of care. Can it work with your existing staff?
Training: Consider how you will train your staff. Who will run the training?
Helpful resources:
Record keeping: Decide how you will approach record keeping so that you can easily identify who has been tested (or taken a kit) and where they are up to in the testing process.
Client data storage: Consider where you will store client results for future reference – do you have client files already, or will you need a system to support this? Record how and when the sample was collected. You may want to consider working with a healthcare provider who has an existing electronic medical record system to ensure the quality of your documentation.
Policies and guidelines: Consider whether you will need to update any existing policies or guidelines within your service to include DBS testing.
Instruction manual: Consider whether you will need an instruction manual. You might be able to get this from your test kit supplier or adapt existing user manuals from other services. Consider the audience of your instruction manual – will it be clinical staff, peers or consumers? Adapt your communication style for the audience.
Consent forms: Make sure you have appropriate consent forms that outline what the testing process is, what the results mean, and possible risks so that your client can make an informed decision.
Confidentiality: Consider how you will keep and maintain confidential records and ensure the privacy of your clients’ information.
Helpful resources:
Who will organise the supplies? Identify who will be responsible for packing and restocking kits.
Who will perform the collection? Identify who will perform the DBS collection – e.g. nurse, physician, other health worker, peer, self-collection, or various providers depending on the situation.
Who will provide the results? Identify who will deliver results to your clients – are there any rules in your region about who is legally able diagnose HCV and report it to your public health unit?
How will you provide the results? Consider how you are going to provide test results to people. Negative results can be delivered either via email, text message, phone call, or in person. Positive results should be delivered in person by a nurse or other experienced healthcare provider who can provide support and information about next steps for treatment and care.
Who will notify positive results to the relevant body? If HCV is a notifiable disease in your region, identify who will be responsible for notifying the relevant body (e.g. your local public health department) of a positive result. In many regions, the lab will notify the relevant body. If DBS testing is not approved in your region as a diagnostic tool, consider how you will link clients into confirmatory RNA testing.
How will you link clients into treatment? Consider how you will link clients into treatment if they receive a positive result.
Training: You may need to consider training the relevant individuals on:
- How to perform the collection
- How to interpret results
- Handling and disposal of infectious substances (fresh blood)
- Infection prevention and control
- Pre- and post-test counselling
- Client education and support
Resources: Consider the tools that will support your staff and clients. This may include:
- Laminated infographics or directions to show the client how the process works
- Consent forms (paper or electronic)
- Client info sheets
- Procedure manuals
Helpful resources:
What will you test for on the DBS card? Discuss with your team/manager and agree on what you will test for – will you also include HBV and HIV testing? Will you provide a point-of-care antibody test, and then use the DBS to confirm positive results?
Who will you test? Will you use DBS testing for all clients, or just for specific populations/situations? If DBS testing is only being used for specific populations, ensure you have a written protocol that records this, and that everyone involved is aware of this. Develop a brief screening questionnaire to establish risk, if you don’t already have one.
When will you offer a test? Decide when to offer a client a DBS test – is there a logical point in your interactions with people at which you can ask whether they would like one?
Where will you collect the sample? Will clients take home kits, or will you only offer collections during clinic visits, outreach etc? Check whether your DBS kit supplier allows for client self-collection. Decide whether you will supply kits to people to perform their own DBS collection at home, or whether you will have staff administer the collection on-site.
For on-site sample collection: Arrange a private area with cleanable disinfected surfaces that are suitable for sample collection and consultation.
For take-home kits:
- Make sure people requesting kits will have access to the information and advice they will need. Ensure this is available in their preferred language. Ensure any instructions are accessible for people who cannot read or have a vision impairment
- Check whether there are any requirements for posting DBS samples and whether you can track the cards in the mail
- Set up a process for keeping track of who has received a DBS kit, when it is complete, and when the results are due
- Consider client privacy – decide what details will appear on the postage envelope
How will you provide results? Consider how you are going to provide test results to people. Will this be different if the result is negative (e.g. phone, text message, email) or positive (e.g. in person). Consider obtaining written consent on how you will provide the client with their results – you could include this in your consent forms.
Linkage to care: Identify the client pathway to receiving care if they get a positive HCV result. Consider whether that pathway will work for most of your clients – will some require extra help? If you’re testing for other diseases on the DBS card (HIV, HBV, etc) you will need to consider the client pathway to care for a positive result in those situations too.
Re-testing: Decide how often you will offer re-testing.
Helpful resources:
Consider using an implementation theory, model or framework: Using an implementation theory, model or framework can help guide the process of translating research into practice, understand and/or explain what influences implementation outcomes and evaluate implementation. Consider using a person-centred care framework when developing your implementation plan.
Select implementation strategies: There are a number of evidence-based strategies (methods, techniques) that you can use to promote and support the implementation and sustainability of DBS testing in your service. Here are a few examples:
- Build a coalition: Recruit and cultivate relationships with partners in the implementation effort. Are there existing local events or other services where you could create a partnership to offer a DBS testing service? For example, local shelters, OST/OAT clinics, outreach services, etc.
- Conduct local consensus discussions: Include local providers and other stakeholders in discussions that address whether the model of care you have selected in relation to DBS testing it is appropriate.
- Identify and prepare champions: Identify and prepare individuals who dedicate themselves to supporting and marketing the implementation to overcome indifference or resistance. These champions can get the word out and increase awareness in your local population.
- Involve clients: Engage or include client and families in the implementation effort to highlight positive experience. Can you create a narrative of positive experiences from people who have taken the test?
- Involve and train peers: Engage or include trained peers in your model of care and in the implementation effort more broadly. Peers can provide support, promote DBS testing and collect DBS samples if they are trained properly.
- Conduct educational meetings: Hold meetings with different stakeholder groups (e.g., providers, administrators, and community, client, and family stakeholders) to teach them about DBS testing.
- Develop and disseminate educational materials: Develop and format toolkits and other supporting materials (in addition to the instruction manual) for stakeholders to learn about DBS testing and for clinicians to learn how to deliver it.
Helpful resources:
- INHSU – Items you might need to order
- INHSU – Peer Support Intervention
- INHSU – Patient Navigation Intervention (coming soon)
Evaluation plan: Plan how you will evaluate the uptake and effectiveness of the intervention. How will you know if the intervention has been useful?

Check with your local public health department, pathology provider, medical microbiologist, hospital laboratory, or try contacting other organisations in your country who have already explored DBS testing.
If they can’t point you in the right direction, contact us and we’ll do our best to help.
Most of the items can be purchased separately if a complete kit is not available. You will need to check with the lab processing the DBS about which cards are approved for use.
Approach testing manufacturers to see if they would like to develop a service. Provide examples of other places that provide DBS kits.
This is highly dependent on the health system you are in.
Check with your local public health department or hospital laboratory, or try contacting other organisations in your country that have already explored DBS testing.
If they can’t point you in the right direction, contact us and we’ll do our best to help.
Contact other services that have successfully implemented DBS testing and ask them for advice. Use your networks to promote use, and ask peers, clients, and staff to promote it.
Rather than seeking funding for a permanent program, consider approaching management for a pilot project. Agree on endpoints and metrics that will objectively show whether there is a benefit from using DBS testing.
Consider link your DBS testing initiative to local, state, national or international HCV elimination goals or strategies.
Contact other services that have successfully implemented DBS testing and ask them for advice. You might not need funding – in many places, DBS tests are subsidised by governments or health departments.
If not, depending on the type of organisation you are in, you may be able to seek grants to support implementation. Also, consider approaching pharmaceutical companies working in HCV.
You will need to train staff in both the proper collection of DBS samples and how to counsel people being tested. Your testing lab may be able to provide training on DBS collection. Public health or a local infectious disease physician may be able to provide training on how to conduct pre- and post-test counselling.
There are a lot of existing resources that you can use – see Resources, Tools and Pro-Forma
Check with your lab. They should be up to date on the literature surrounding ideal conditions for sample storage.
Run a few simulated clinic visits, where they can practice the process.
Talk to the hepatology, gastroenterology, or infectious diseases department at your local hospital – they should be able to suggest some options. There may be community prescribers who your clients can easily access.
Contact your local public health unit to understand whether there are processes in place for the notification of results from DBS tests.
Consider ensuring a pathway for confirmatory testing is in place and demonstrate how DBS assists to reduce barriers by engaging clients in a care pathway
Connect with a healthcare provider in your region. They might be able to provide a medical directive to allow you to order and/or perform the test under their supervision, or with their sign off.
Videos
Resources
- INHSU resource: Items you might need to order
- INHSU resource: How to collect a DBS sample
- INHSU resource: DBS samples – examples of valid and invalid samples
- INHSU resource: How to interpret a DBS result
- CDC Shipping Guidelines for Dried-Blood Spot Specimens
- WHO Guidance on regulations for the transport of infectious substances 2021-2022
Frequently asked questions 
Are dried blood spots infectious?
According to WHO, DBS samples are not considered to pose a health risk. Samples should be handled with care in order to avoid contamination or degradation of the sample, but they are not considered to be an infectious substance.
While the samples are not infectious, WHO guidelines say they must be transported using a basic triple packaging system. You can find out more about packaging specifications from the WHO Guidance on regulations for the transport of infectious substances (see pg. 11).
Is the sensitivity for detecting HCV RNA from DBS lower than from EDTA plasma- and serum-collected samples?
The sensitivity for detecting HCV RNA decreases with the amount of sample tested, and this volume is lower when using DBS (just a few drops) in comparison with plasma or serum obtained from venous blood. This could potentially lead to false negative results in cases with very low levels of RNA, which may be present in approximately 5% of all viremic cases. However, the risk of obtaining false negative results is outweighed by the benefits of increasing access to diagnosis for people who would otherwise have fewer testing options through mainstream services.
Got a question?
Contact us and we’ll do our best to help.
What does the evidence say?
Impact of DBS testing on HCV testing and linkage to treatment:
Title |
Author |
Year |
Link |
| Interventions to enhance testing and linkage to treatment for hepatitis C infection: a systematic review and meta-analysis | Cunningham EB, et al. | 2021 | Read the paper |
| Hepatitis Service Provision at HMP Birmingham: Progressing a Previous Service Improvement Plan | Arif T. | 2018 | Read the paper |
| A quasi-experimental evaluation of dried blood spot testing through community pharmacies in the Tayside region of Scotland | Radley A, et al. | 2017 | Read the paper |
| Multidisciplinary managed care networks—Life-saving interventions for hepatitis C patients | Tait J, et al. | 2017 | Read the paper |
| Integrating community pharmacy testing for hepatitis C with specialist care | Buchanan R, et al. | 2016 | Read the paper |
| The effect of introducing point-of-care or dried blood spot analysis on the uptake of hepatitis C virus testing in high-risk populations: A systematic review of the literature | Coats J, et al. | 2015 | Read the paper |
| A stepped wedge cluster randomized control trial of dried blood spot testing to improve the uptake of hepatitis C antibody testing within UK prisons | Craine N, et al. | 2014 | Read the paper |
| Rise in testing and diagnosis associated with Scotland’s Action Plan on Hepatitis C and introduction of dried blood spot testing | McLeod A, et al. | 2014 | Read the paper |
| Improving blood-borne viral diagnosis; clinical audit of the uptake of dried blood spot testing offered by a substance misuse service | Craine N, et al. | 2009 | Read the paper |
| Increasing the uptake of hepatitis C virus testing among injecting drug users in specialist drug treatment and prison settings by using dried blood spots for diagnostic testing: a cluster randomized controlled trial | Hickman M, et al. | 2008 | Read the paper |
Sensitivity and specificity for dried blood spot testing:
Title |
Author |
Year |
Link |
| Diagnostic accuracy of assays using point-of-care testing or dried blood spot samples for the determination of HCV RNA: a systematic review | Catlett, B | 2022 | Read the paper |
| Evaluation of the diagnostic accuracy of laboratory-based screening for hepatitis C in dried blood spot samples: A systematic review and meta-analysis | Vázquez-Morón, S | 2019 | Read the paper |
| Diagnostic accuracy of detection and quantification of HBV-DNA and HCV-RNA using dried blood spot (DBS) samples – a systematic review and meta-analysis | Lange B, et al. | 2017 | Read the paper |
| Diagnostic accuracy of serological diagnosis of hepatitis C and B using dried blood spot samples (DBS): two systematic reviews and meta-analyses | Lange B, et al. | 2017 | Read the paper |
| Dried blood spots as sample collection method for HCV antibody: a systematic review and meta-analysis | Lange B, et al. | 2015 | Read the paper |
Optimal collection, transport and storage conditions:
Title |
Author |
Year |
Link |
| Dried Blood Spots for Global Health Diagnostics and Surveillance: Opportunities and Challenges | Lim M. | 2018 | Read the paper |
| Dried blood spot in the genotyping, quantification and storage of HCV RNA: a systematic literature review | Greenman J, et al. | 2014 | Read the paper |
Implementation theories:
Title |
Author |
Year |
Link |
| Making sense of implementation theories, models and frameworks | Nilsen P. | 2015 | Read the paper |
| A refined compilation of implementation strategies: results from the Expert Recommendations for Implementing Change (ERIC) project | Powell B, et al. | 2015 | Read the paper |
| Effective Practice and Organization of Care (EPOC) | Shepperd S, et al. | 2015 | Read the paper |
Photos used with permission from the Kirby Institute, UNSW, Sydney, Australia